Properties of Fluids
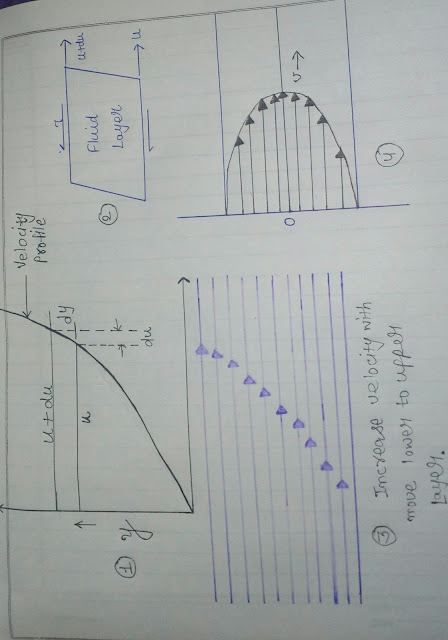
We know about fluid and students every fluid(liquids, vapours, gases) has definite properties which help us to describe its physical condition. Such properties are called properties of fluid. It also helps us to analysis fluid flow problem. PROPERTIES OF FLUIDS There are main properties of fluids following :- 1. Density or Mass Density:- It is the ratio of mass of a fluid to its volume. It is de noted by ρ( ro ). Mass Density,. p = Mass of fluid/volume of fluid =m/v SI Unit of density or Mass Density is Kg/cubic meter. The density of water is 1000kg/cubic meter or 1 gm/cubic centimeter. 2. Specific Weight:- It is defined as the ratio of the weight of a fluid to its volume. It is denoted by 'w' and is also called weight density. w =. Weight of fluid/volume of fluid = mass of fluid ×g/volume of fluid = Density of fluid× g. =. ρg Unit of specific weight i